Abstract
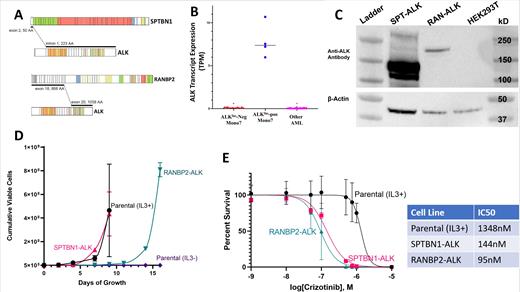
Monosomy 7 (Mono7) AML is one of the most established adverse prognostic markers in AML with over four decades of data on response and outcome in varied clinical trials. However, despite intensive and myeloablative therapies, outcome for this subtype of AML remains dismal with no meaningful advances in therapy in decades. As part of our recently completed discovery efforts in childhood AML (TARGET Pediatric AML; TpAML), we interrogated the transcriptome of 1068 children and young adults treated on COG AAML1031, including 28 cases of Mono7. We discovered novel cryptic fusions involving the Anaplastic Tyrosine Kinase (ALK) gene that were exclusively seen in 4 of 28 Mono7 patients (14.3%) and not detected in the remaining 1064 patients (p<0.001). These ALK fusions (ALK fus) included SPTBN1-ALK (n=3) or RANBP2-ALK (n=1) (figure 1A) and all 4 cases included the entire kinase domain of the ALK gene. Expression of ALK transcript was evaluated in Mono7 patients with and without ALK fus to determine whether these fusions lead to induction/upregulation of ALK. As demonstrated in figure 1B, the only patients with any ALK expression were those with ALK fus positive Mono7.
Expression of the resultant onco-proteins of the ALK fus was confirmed by transfection of parental HEK293T cells with the oncofusion and western blot analysis of the protein lysates. As shown in the western blot in figure 1C, immunoblotting of the lysates from ALK fus cells with ALK antibody confirmed expression of the appropriate ALK+ fusion oncoprotein. Under experimental conditions, fusion involving SPTBN1-ALK appeared to generate higher levels of the fusion onco-protein compared to that of RANBP2-ALK fusion. Confirmation of the fusion onco-protein expression further validates the possibility of the functional significance and potential targetability of these fusions.
The ALK gene codes for a receptor tyrosine kinase belonging to a family of protein kinases linked to unregulated cell growth which play a key role in CNS development. Alterations of the ALK gene are commonly seen in Non-Small Cell Lung Cancer (NSCLC), Anaplastic Large-Cell Lymphomas, and Neuroblastomas. We further inquired about the functionality of the ALK fus and whether they may confer cytokine-independent proliferation. The ALK fus were cloned from patient samples into pCSII, a direct GFP-tagged lentiviral backbone under the Ef1α promoter; followed by Sanger sequencing to verify the insert. IL-3 dependent Ba/F3 cells were transduced with the GFP tagged lentiviral vectors containing the SPTBN1-ALK or RANBP2-ALK fusion transcripts.
Transduced cells were sorted to GFP homogeneity and growth parameters evaluated post-IL-3 withdrawal. In contrast to the parental line, which rapidly died in the absence of cytokines, cells expressing either SPTBN1-ALK or RANBP2-ALK sustained growth and rapidly proliferated in cytokine-free media, suggesting a conferred transformation event by the fusions, figure 1D. SPTBN1-ALK fusion appears to show more transforming potential with more rapid cytokine-free proliferation compared to that of RANBP2-ALK fusion. The observed differential growth pattern with the two ALK fus correlates with the differential expression of the two oncoproteins as presented above.
We then sought to evaluate the efficacy of Crizotinib as a potential therapy for aberrant ALK fus positive Mono7 AML. Crizotinib has been FDA-approved and demonstrated efficacy in treating ALK positive NSCLC. ALK fus -positive cells were treated with varying doses of Crizotinib in cytokine-free media and cell viability and cell death were determined with the Promega Cell Titer Glow assay after 48 hours in culture. Fusion positive cells show sensitivity to Crizotinib with IC50s of 144nM and 95nM for the SPTBN1-ALK or RANBP2-ALK fusions, respectively.
Here we demonstrate experimental data that when newly discovered cryptic ALK fus in Mono7 AML are functional (translation to fusion onco-protein, conferring cytokine independence) and show susceptibility to the ALK inhibitor crizotinib. This data may support effective therapeutic targeting of a subset of this highly refractory AML.
Hylkema: Quest Diagnostics Inc: Current equity holder in publicly-traded company; Moderna: Current equity holder in publicly-traded company.
Crizotinib is a first-generation ALK inhibitor that is FDA-approved for the treatment of Non-Small Cell Lung Cancer, Anaplastic Lymphoma, and Neuroblastoma.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal